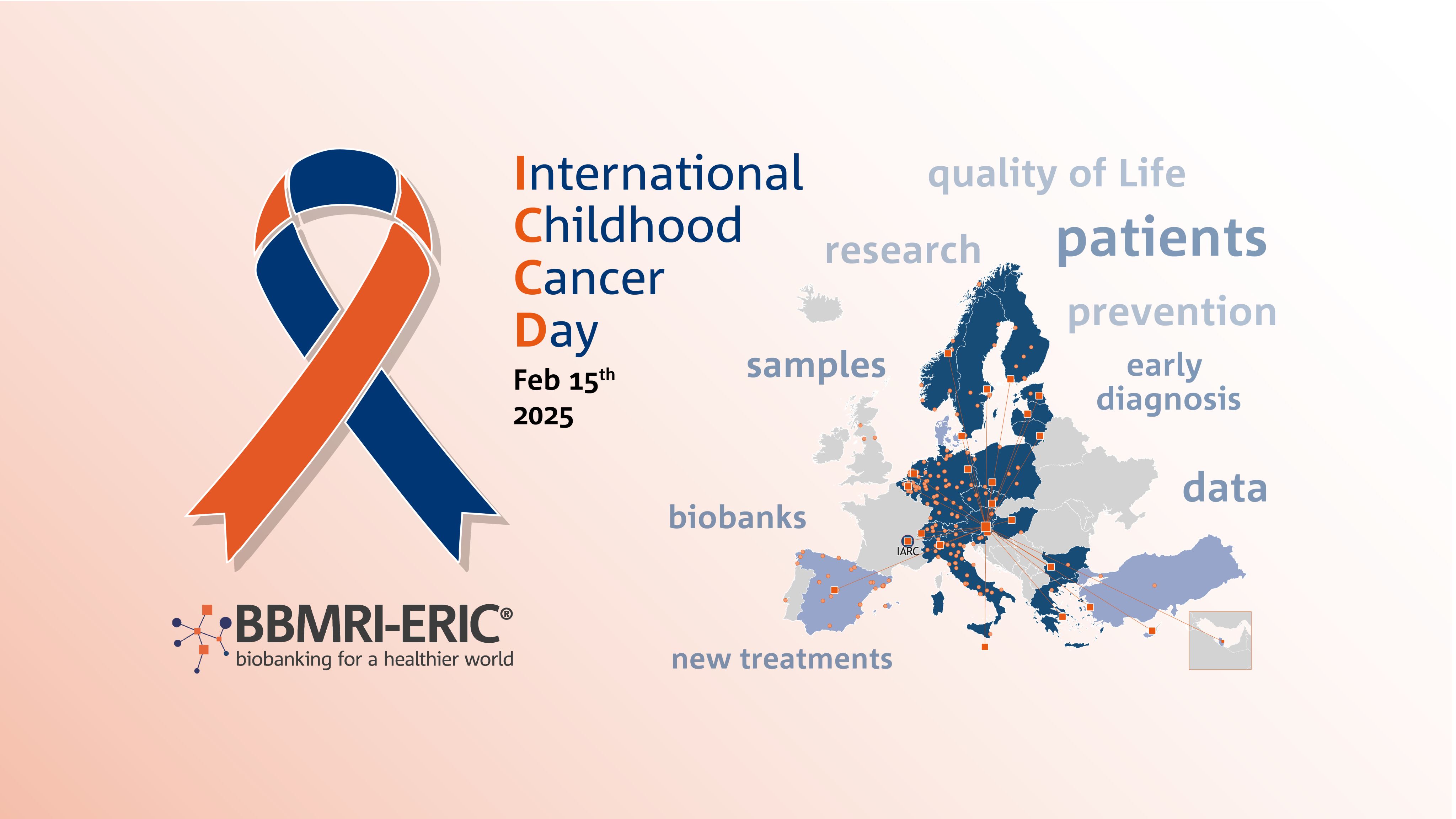
Feb 15th was International Childhood Cancer Day. To shine a spotlight on paediatric cancer research, we invite you on a biobank journey across Europe.
We will visit seven BBMRI-ERIC biobanks that work towards the shared goal to provide better treatments and care for children of all age with cancer. Our journey will take us to Sweden, Latvia, the Netherlands, Germany, Hungary, and a global organisation (IARC/WHO) to highlight different aspects of excellent paediatric cancer research.
Biobanks are vital in paediatric oncology by building, sustaining and sharing repositories of biological samples for research. These samples help us better understand cancer biology, develop new treatments, and improve diagnostic methods.
The UN Rights of the Child states:
“Children have the right to the enjoyment of the highest attainable standard of health and facilities for the treatment of illness and rehabilitation of health.”
In the age of Personalised Medicine, this includes recognising that children have different medical needs when it comes to cancer treatment. Here, specialised paediatric sample and data collections are invaluable resources for the development of the best possible treatment and care for children with cancer. 🎗️🧡

We start our journey up north in Sweden.
The Swedish Childhood
Tumor Biobank (BTB)
Frozen patient samples in bar-coded sample tubes on dry ice. (c) BTB
Frozen patient samples in bar-coded sample tubes on dry ice. (c) BTB
BBMRI-ERIC paediatric biobank case study 1: The Swedish Childhood Tumor Biobank (Barntumörbanken, BTB)
In Sweden, approx. 350 children are diagnosed with cancer every year. Today more than 85% of the patients will survive, but cancer is still one of the major medical causes of childhood death. Survivors can suffer from chronic health problems throughout their lives. We therefore need deeper, more detailed biological knowledge of cancer development in children to increase survival and improve their quality of life.
The Swedish Childhood Tumor Biobank (Barntumörbanken, BTB) collects tissue samples and genomic data from paediatric patients diagnosed with central nervous system (CNS) and other solid tumours. Essential prerequisites for the responsible use of these samples for research is that donors give their informed consent and that the samples are stored safely for years to come. Regarding regulatory, logistical and organisational issues, the BTB is supported by the Biobank Sweden (Biobank Sverige) and their regional biobank Stockholm Medical Biobank. The work at the BTB is financially supported by the Swedish Childhood Cancer Fund.
In the BTB, more than 2,400 cases are registered with approximately 60,000 samples. Thousands of these cases have already been characterised via whole-genome sequencing (WGS) or whole-transcriptome sequencing (WTS). The samples and associated data provided to the scientific community by the BTB represent invaluable and precious resources for cancer research. With these samples, researchers can increase our knowledge of the biology, diagnosis, and treatment of childhood cancers.
To date, these samples and data at the BTB have supported more than 20 different research projects and BTB is assisting several clinical studies with sample logistics, regulatory support and data analysis/interpretation. The more we learn about the behaviour of tumours, we can identify more risk factors early on and develop prevention strategies.
Resources for researchers/ patients/citizens:
The BTB:
https://www.barntumorbanken.se/
Swedish technologies supporting cancer research: https://www.scilifelab.se/
Biobank Sweden, a national infrastructure for biobanking and the Swedish node of BBMRI-ERIC:
https://biobanksverige.se/en/a-national-infrastructure-for-biobanking/
Swedish Childhood Cancer Fund: https://www.barncancerfonden.se/en/
Image: Lab samples in the BTB (blood and tumor sample on dry ice). © Swedish Childhood Tumor Bank


Now, we'll travel on to Riga, Latvia!
The Latvian Pediatric Cancer Initiative
Lysate of white blood cells. (c) LBMC
Lysate of white blood cells. (c) LBMC
BBMRI-ERIC paediatric biobank case study 2: The Latvian Pediatric Cancer Initiative
The Latvian Pediatric Cancer Initiative launched in 2019 as a collaboration between the Latvian Medical Research & Study Centre (LBMC) and the Children's Clinical University Hospital. The initiative has now collected samples from over 400 paediatric cancer patients, which reflects nearly all cases in Latvia from 2019 to 2025.
These samples of tumour and normal tissues are characterised using whole-genome sequencing (WGS) or RNA-sequencing to characterise their genomic profiles. The goal is to find unknown genetic alterations and signalling pathways that drive paediatric cancers and to use this information for more precise diagnosis and personalised treatments.
Industry support and international collaborations with, for example, the St. Jude Children’s Research Hospital (Memphis, Tennessee) further strengthen state-of-the-art data analysis strategies to find reliable biomarkers that help tailor treatment strategies to individual patients.
The body’s response to cancer can never be viewed as an isolated process. Therefore, LBMC initiated a study on the role of the gut and oral microbiome in paediatric cancers. Factors like antibiotics, chemotherapy, radiation therapy, and dietary changes can disrupt the microbiome composition of a patient which affects their immune response, infection susceptibility, treatment outcomes, and the long-term effects of cancer therapies.
The project aims to establish a comprehensive microbiome sample collection within the national biobank. These samples will allow tracking microbiome changes at key treatment stages, identify microbiome-derived markers, and characterise disease-specific microbiome signatures by comparing samples with those of healthy individuals.
Resources for researchers/ patients/citizens:
The Latvian Biomedical Research & Study Centre (LBMC):
https://biomed.lu.lv/home/
Children's Clinical University Hospital: https://www.bkus.lv/en
Images:
Lysate derived from a patient’s white blood cells at the LBMC. © LBMC
A sequencer at the LBMC. © LBMC

Our next stop: Utrecht in the Netherlands.
The Princess Máxima Center
for Pediatric Oncology
Biobank staff pulling frozen patient samples out of a liquid nitrogen tank. (c) Princess Máxima Center for pediatric oncology
Biobank staff pulling frozen patient samples out of a liquid nitrogen tank. (c) Princess Máxima Center for pediatric oncology
BBMRI-ERIC paediatric biobank case study 3: The Biobank at the Princess Maxima Center for Pediatric Oncology
The Biobank at the Princess Máxima Center for Pedriatic Oncology in Utrecht stores samples of children treated at the Center. Over 5,000 (former) patients have given their permission to store tissue and data in the biobank and by this means directly support cancer research. The Princess Maxima Center brings together cancer research, biobanking and clinical treatment in one place to enable the best possible support for children with cancer.
After taking samples for routine diagnostics, parts of the sample material can be stored for future research provided that informed consent for its use has been given by the patient. These biomaterials (tissue, blood, bone marrow, etc.) and isolated DNA/RNA together with genetic, and imaging data, are stored and curated in the central Máxima Biobank together with associated medical data, including questionnaires about the quality of life.
With this wealth of information, researchers can link the biology of the tumour to how well a certain treatment works or predict which children will be at higher risk of fatigue. Research with these samples thus directly helps to evolve treatment plans and improve the quality of life for the young patients.
A very recent example of how biobank samples at the Princess Máxima Center drive cancer research, was published 2024 in Nature Communications. In this study, researchers used patient-derived tumour organoids (“mini-tumours”, formed by in vitro cultured clusters of tumour cells derived from isolated samples) to screen for novel drugs to treat malignant liver tumours. Currently, treatment of these tumours combines chemotherapy with surgery and often includes a liver transplant which makes live-long immune suppression necessary for the children. Alternative and more personalised treatments could reduce or avoid these harsh interventions for young patients.
Resources for researchers/ patients/citizens:
Princess Maxima Center: https://research.prinsesmaximacentrum.nl/en/core-facilities/biobank
More info on the cited study on "Liver tumor organoids point to potential treatment": https://research.prinsesmaximacentrum.nl/en/news-events/news/liver-tumor-organoids-point-to-potential-treatment
Image:
Biobanking staff at the Princess Máxima Center working with samples stored in liquid nitrogen. © Princess Máxima Center for pediatric oncology


From Utrecht to Lyon.
The International Agency for Research on Cancer of the WHO (IARC/WHO)
Biobank staff working a liquid nitrogen tanks. (c) IARC/WHO
Biobank staff working a liquid nitrogen tanks. (c) IARC/WHO
BBMRI-ERIC paediatric biobank case study 4: The IARC/WHO paediatric cancer collection
Research thrives on trans-national collaborations. The International Agency for Research on Cancer (IARC) is the specialised cancer research agency of the World Health Organization (WHO). IARC/WHO focuses on promoting international collaborations, especially in low- and middle-income countries (LMICs), as there are dramatic differences in cancer survival for children between high- and low-income regions.
To support cancer research, IARC/WHO has built an extensive collection of childhood cancer samples. This collection, located at the IARC/WHO biobank, provides the foundation for many ongoing scientific investigations. Currently, the organisation is running a major international study in multiple countries together with the International Initiative for Pediatrics and Nutrition (IIPAN) at Columbia University Irving Medical Center (New York, USA). The study aims to understand the connection between nutrition, childhood cancer and the microbiome.
A recent systematic review, for example, particularly addressed the connection between “Early Life Nutrition Factors and Risk of Acute Leukemia in Children” (Kintossou et al., 2023). Acute leukaemia commonly occurs in young children with peak incidence at the age of 2–5 years, but the reasons are unclear and knowledge about risk factors is limited. Therefore, the ongoing research is building towards greater clarity for the molecular and biological mechanisms underlying childhood cancer and survival, but also as the first step towards a global strategy for building clinical capacity and advancing research in the context of malnutrition and children with cancer in LMICs.
Resources for researchers/ patients/citizens:
Kintossou et al.’s Leukemia paper: https://pmc.ncbi.nlm.nih.gov/articles/PMC10489830/
Global strategy paper: https://academic.oup.com/jncimono/article/2019/54/149/5567555
Role of maternal diet and risk of childhood leukaemia: https://www.mdpi.com/1660-4601/20/7/5428
IARC/WHO nutrition research:
https://www.iarc.who.int/news-events/iarc-study-on-nutrition-and-childhood-cancer-noncommunicable-diseases-wha/
IIPAN:
https://www.pediatrics.columbia.edu/about-us/divisions/hematology-oncology-and-stem-cell-transplantation/international-initiative-pediatrics-and-nutrition-iipan
Images: IARC/WHO biobank staff at work. © IARC/WHO


Next stop: Augsburg
The Augsburg Central BioBank (ACBB)
ACBB biobank staff preparing samples for analysis. (c) ACBB
ACBB biobank staff preparing samples for analysis. (c) ACBB
BBMRI-ERIC paediatric biobank case study 5: The Augsburg Central Biobank (ACBB)
Biobanks are often linked to clinics or university clinics as this allows tight coordination between taking and managing samples as well as providing care for patients and doing research. Since 2019, the Augsburg Central Biobank (ACBB) has stored 147 paediatric cancer samples. Every patient is different and has different genetic predispositions, health status or microbiome composition. Thus, every single sample helps to represent this diversity in research. Integrating these individual characteristics of a patient not only helps to find the best possible treatment strategy for this individual person but increases our ability and knowledge to apply personalised treatment strategies for all patients via Personalised Medicine.
The ACBB processes tumour tissue samples from minors and stores them as fresh frozen samples at temperatures below -150°C. These low temperatures are essential to preserve the integrity of these samples for many years. Collecting and storing these samples is thus a direct investment in cancer research for decades to come. If possible, the ACBB also stores tissue samples from healthy patients as those are strongly needed as references for research projects.
In its early phase, the ACBB collected cryo-preserved tissue samples including solid tumours of the lung or colon, but the spectrum of collected cancer and sample types is constantly increasing in tight coordination with the clinicians. Now, the ACBB also collects liquid samples (e.g., blood plasma, serum, urine), isolated DNA or mononuclear cells. This includes samples that are taken routinely for example for diagnostic purposes but also samples that are collected in dedicated studies.
The ACBB also stores paediatric samples from patients with Rhabdoid tumours (e.g. blood and tissue samples) for the European Rhabdoid Registry. By participating in trans-national projects, infrastructures like BBMRI-ERIC and sample/data sharing initiatives, also smaller collections stored in local biobanks support research across the continent. This multiplies the impact these samples can have for research and thus for patients.
Resources for researchers/ patients/citizens:
ACBB:
https://www.uk-augsburg.de/einrichtungen/institute/institut-fuer-pathologie-und-molekulare-diagnostik/biobank/ueberblick
European Rhabdoid Registry: https://www.gpoh.de/kinderkrebsinfo/content/fachinformationen/studienportal/onkologische_studien_und_register/eu_rhab_register/index_ger.html
Images:
ACBB Biobank staff preparing samples for analysis. © ACBB
ACBB Biobank staff taking samples put of a -80°C freezer. © ACBB


One more stop in Germany: Aachen
The RWTH centralized
BioMaterial Bank (cBMB)
Biobank staff pipetting samples under a laminar flow box. (c) RWTH cBMB
Biobank staff pipetting samples under a laminar flow box. (c) RWTH cBMB
BBMRI-ERIC paediatric biobank case study 6: The RWTH centralized Biomaterial Bank (RWTH cBMB)
The cBMB at the University Hospital Aachen operates in close collaboration with the Department of Pediatric Hematology and Oncology. Close interdisciplinary exchange and collaboration is a crucial prerequisite for the design and successful completion of large-scale clinical studies.
Prof. Kontny, Head of the Section for Pediatric Hematology, Oncology and Stem Cell Transplantation at the University Hospital Aachen, summarises the essential role of biobanks as follows: "Modern biobanking is a prerequisite for my pediatric oncology research”
Currently, the department conducts a multicenter, prospective phase 2 clinical trial in children and adults with nasopharyngeal carcinoma. The goal of this trial is to reduce the frequency and severity of late effects, especially in younger patients.
The study examines if integrating immune checkpoint inhibitors into the treatment regime of these patients allows a reduction of the radiotherapy intensity and thus side effects of the treatment. Immune Checkpoint Blockade therapy uses specific inhibitors that prevent that the activity of the immune system is lowered due to inhibitory immune checkpoint molecules. This therapeutic intervention strengthens the natural capability of the patient’s immune system to act against the cancer cells.
During this clinical trial, biomaterial such as tumour tissue, blood and saliva is collected at different time points and stored at the cBMB. So far, 2,524 aliquots of biomaterials have been sampled in this project. The outcome will help to improve the quality of life of young cancer patients during and after their treatment.
Due to its long-standing participation in the German Biobank Alliance (GBA), the cBMB (scientific director: Prof. Dahl) can operate at highest quality standards in the processing and storage of these biomaterials.
As pan-European infrastructure organisation, BBMRI-ERIC further expands this level of cooperation and scientific exchange by connecting currently more than 400 biobanks across Europe and beyond.
Resources for researchers/patients/citizens:
The RWTH cBMB:
https://buff.ly/3CU1Ejg
Protocol of the prospective multicenter phase 2 trial: https://buff.ly/4gCOGnH
The German Biobank Alliance:
https://www.bbmri.de/ueber-gbn/german-biobank-alliance/
Image: Biobank staff at the cBMB handling patient samples. © RWTH cBMB


Last but not least: Hungary
The Hungarian Sample Collection of Pediatric Diseases (SCOPEDIS)
Colour-coded sample storage in a freezer. (c) SCOPEDIS
Colour-coded sample storage in a freezer. (c) SCOPEDIS
BBMRI-ERIC biobank case study 7: The Hungarian Sample Collection of Pediatric Diseases (SCOPEDIS)
As early as 1971, the Hungarian Pediatric Oncology Network started a national registration system for all children diagnosed with haematological and solid tumours. To support this, the Hungarian Childhood Cancer Registry was founded to collect and store structured patient data for research.
In 2022, the SCOPEDIS (Sample Collection of Pediatric Diseases) Biobank project started. Its aim was to set up a quality-assured infrastructure for the systematic collection, processing, storage, and open distribution of samples and associated data across the networks’ eight treatment centres in Hungary.
So far, approximately 900 samples and detailed associated datasets have been collected from paediatric cancer patients. The samples and pseudonymised data are obtained at each collection centre from all children diagnosed with malignancies or pre-malignant tumours. The SCOPEDIS Biobank manages a diverse range of samples, including blood, bone-marrow aspirates, and solid tumour tissue, all accompanied by pre-analytical and clinical information.
As prerequisite for using the samples for research, the biobank staff thoroughly documents and monitors storage conditions and aims to assess the quality of the samples before distribution via e.g., DNA/RNA, total proteome quantification, or tissue integrity analysis. To uphold the highest quality standards for these precious samples, the SCOPEDIS Biobank adheres to the requirements of ISO 20387 norm and international best-practice guidelines.
A biobank cannot support research unless there are patients willing to give their samples and the informed consent to use them. To strengthen the bond between the research community and participating patients and families, the network has set up a dedicated website.
This website provides transparent information about processes like sample and data access and serves as a communication platform for participants and researchers. This is a prime example of an outreach initiative to raise public awareness and understanding for the need and social benefit of modern biobanking for research.
Resources for researchers/ patients/citizens:
The SCOPEDIS website:
www.biobank.org.hu
The Hungarian Pediatric Oncology Network: https://www.bbmri.hu/biobanks/hungarian-pediatric-oncology-network-scopedis-biobank/
Images: A low-temperature freezer with colour-coded biobank samples at the SCOPEDIS. © SCOPEDIS
A small storage/freezing box for barcoded sample tubes. © SCOPEDIS