'Bridging the Gap between Researchers and Research Infrastructures'
A report from the EOSC-Life Workshop
Utrecht, 13-14 June 2023

This story is best viewed in landscape.
Ambition vs Reality
Joint life sciences research infrastructures (RIs) like EATRIS, BBMRI-ERIC and ELIXIR, develop and provide services for specific research communities helping to solve bottlenecks and push forward the frontiers of science.
In practice, there are some challenges to make the connection between developers and users. Often, the user community is unaware of the services available to them, and the RIs need to stay in touch with potential users to get a clear picture of their actual needs.
Bridging this gap is one of the great challenges of modern life sciences research, to be able to fully exploit the vast – and quickly growing – amount of health and life sciences research data that is being generated.
Two-Day Workshop
On Tuesday 13 and Wednesday 14 June, data professionals gathered in the beautiful and historic Paushuize in Utrecht to address this challenge to bridge the gap between researchers and the life science & health research infrastructures.
The two-day event was jointly organised by Lygature, EOSC-Life, and Health-RI.
"With this event we hope to reach out to a larger community to get more engagement between research communities and researchers."
Jan-Willem Boiten - Lygature - introduces the workshop


Change is Coming
Traditional RIs offer access to a large service, usually around an instrument. Historically, infrastructures are to get researchers the tools they need. Changes in the research policy landscape, for example the development of the European Health Data Space (EHDS), EOSC, and the push towards Open Science, have caused the role of RIs to change. Nowadays, they often take the role of helping scientists explore the European research landscape and showing the tools and infrastructures available to them.
Niklas Blomberg, Director of ELIXIR, Coordinator of EOSC-Life, and keynote speaker on the first day, gave an example. "Policy makers want to make sure that public money on research is spent wisely", he told the audience, "but to also deal with the reproducibility crisis. It is worrying for funders that a large part of research is not reproducible. How can individual scientists be helped with these policy requirements from funders?"
ELIXIR and other RIs have translated this need into a set of tools to help researchers. But these tools can be either overwhelming – where to start? – or underwhelming, e.g. too generic. Blomberg explained:
"For that reason, our infrastructures are distributed in order to be able to organise the support locally, so, close to the researcher."
In the UK, for example, there is an initiative of FAIR data stewards across science disciplines, to guide researchers into making their data findable, accessible, interoperable, and reusable by design.


Community-driven Effort
A speaker in the FAIR data management & implementation theme, Munazah Andrabi from the UK-branch of ELIXIR, is Co-Lead of this data stewardship community and Co-Editor of the Research Data Management (RDM) toolkit, promoting researchers to generate their data FAIR by design. She addressed the challenge of funding for the RDM toolkit coming to an end, something that many of these tools are facing at some point.
"We developed the toolkit as a community-driven effort from the beginning, as to be able to sustain it without any project funding. We will never run out of users and contributors."
Hear Munazah talk about the RDM Toolkit
Three Tips for Data Stewardship
Mijke Jetten, Community Manager of data stewardship at Health-RI and ELIXIR-NL in The Netherlands, shared her optimism of the growing number of data stewardship communities, exchanging best practices and information. "They were organised from the life sciences in 2017, but have grown broader now", she said.
"There are a lot of initiatives, ranging from global to very local within institutes.’ Covid had a positive effect here, since the meetings shifted from face-to-face to online, boosting attendance and participation."
Mijke shares three tips with us:
1. Engage with your audience.
2. Take along the perspective of the organisation as well.
3. Make sure you have a community manager on board.
Listen to Mijke's three tips
Long-term Funding Needed
Steve Canham, Data and IT Consultant at the European Clinical Research Infrastructure Network (ECRIN), showed the audience a glimpse of the Clinical Research Metadata Repository (MDR). This tool tries to address the problem of finding and getting access to clinical trial data resources.
With this very ambitious goal, and being not the only initiative exploring this issue, Steve was very open in sharing the many challenges his team face, including messy data, poor access to high-quality IT staff, and long-term funding issues. Currently, the MDR is in beta, but already has access to over 800.000 studies from various sources, including YODA and PubMed. Steve explained:
"People tend to focus on the frontend, the portal, but most of the work is at the backend."
Steve Canham on funding



Visions of Personalised Treatment for Rare Diseases
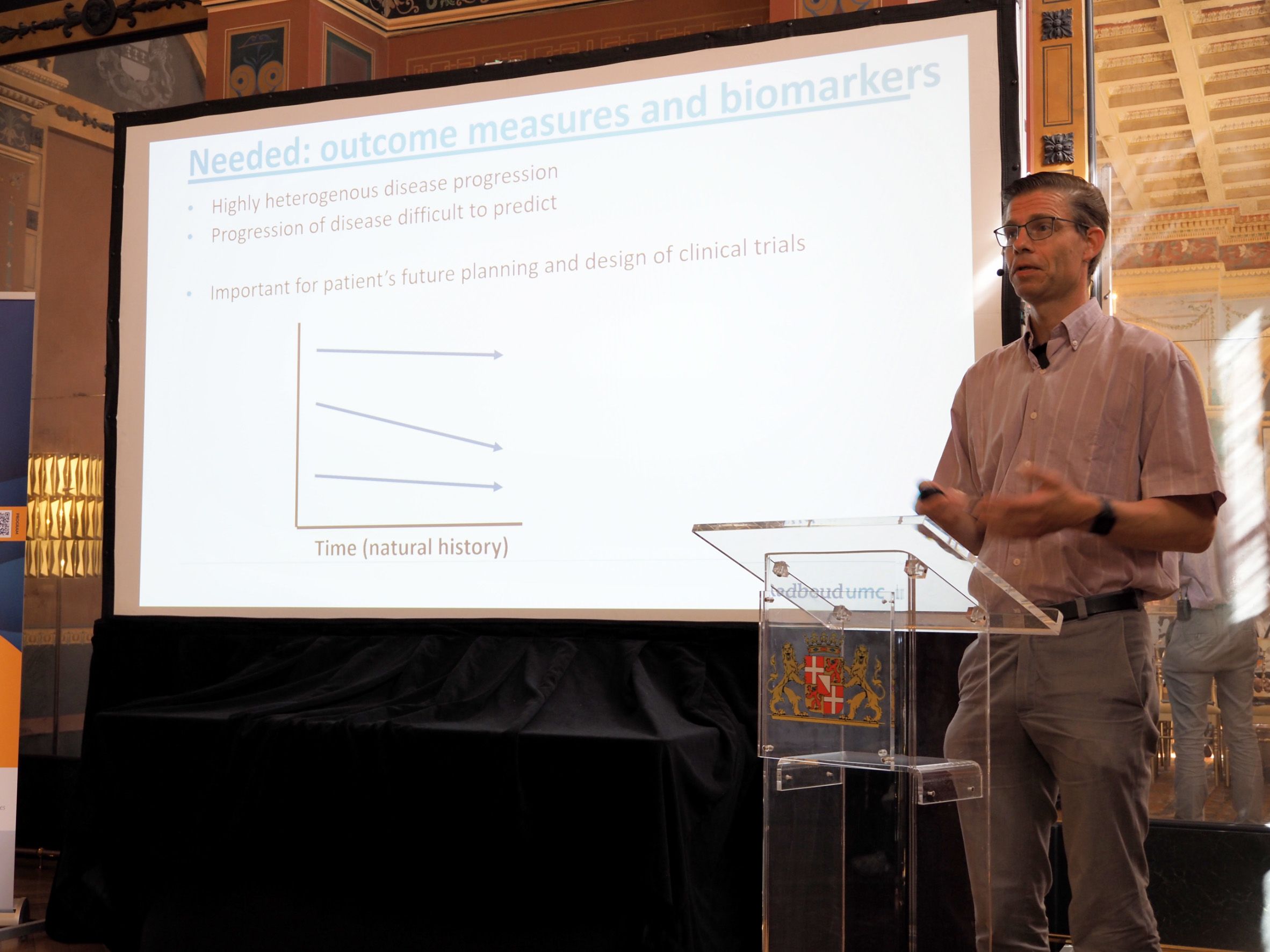
Second-day keynote lecturer Prof. Peter-Bram ‘t Hoen, Chair of Bioinformatics from the Radboudumc in The Netherlands, painted a very practical picture of current health data challenges by sharing his experiences with FAIR patient registries and omics data for modelling disease trajectories.
He focuses on Myotonic Dystrophy Type 1 (MDT1), a rare multisystemic disease affecting the muscles and other body systems. Due to the heterogeneous nature and rarity of the disease, reliable prediction with robust outcome measures is key for meaningful high-quality clinical trial design.
Peter-Bram used machine learning to predict which patients in the OPTIMISTIC clinical trial cohort would benefit from a new therapy on which outcomes were measured. Many clinical and molecular parameters were tested and evaluated; new leads are being followed-up on.
His take-home message was that for rare diseases even more so than for common diseases, it is key to be able to cross-reference high-quality clinical and molecular data, in accessible databases and biobanks across Europe. He is therefore a strong supporter of making data FAIR at the source and accessible through federated systems.
In the vision of the European Joint Programme on Rare Diseases (EJP RD), all rare disease patients in Europe are registered in FAIR registries. Peter-Bram's personal vision goes even further, striving for personalised treatment for rare diseases like MDT1 through prediction of response to treatment.
Listen to Peter's full keynote lecture
ELSI: Getting the Questions Clear
On the topic of sensitive data sharing, Michaela Th. Mayrhofer, Head of ELSI Services and Research at BBMRI-ERIC, talked about the service that provides expert support in ethical, legal, and societal issues that arise in life sciences research. She told her audience in Utrecht the matter of ethics is more complex than many researchers realise, so it needs a team with diverse expertise and experience to aid on the matter of ELSI.
"The dream of a single ethics expert who can address all questions is a not-existant trait."
She also addressed the fact that everyone agrees ethics are very important, but its incorporation in projects is often overlooked or lacks proper funding. Also, availability and accessibility are often lacking, which Mayrhofer’s team tries to solve by building an ELSI knowledge base, developing tools and practical guidelines.
Currently, the BBMRI ELSI team participates in 23 different projects, that has led to extensive publications. However, "the interesting part is the translation in practice," Mayrhofer says. To accommodate this, there is a BBMRI helpdesk dealing with questions on a case-by-case basis.
Often it takes more time to get the question clear than to actually answer it."
She uses the 3:1 ratio, translating to three hours for finding out the question and one hour to answer it.
Michaela on the critical role of ELSI for Life Sciences research
Patient Engagement: Doing it Right
More tools and practical guidelines were presented by Florence Bietrix from the Patient Engagement Resource Centre (PERC) at EATRIS. "Patient engagement is a lot more than just being part of a clinical trial," she argued. "It can start in the preclinical phase by asking for patient opinions and views on anticipated or performed research." From bed to bench side and back: there are many actors involved that relate to patient engagement.
Why patient engagement is so important in medical and life sciences research, can be summarised in five points:
1. It brings the subject at the heart of the process.
2. It brings trust.
3. It brings quality and relevance.
4. It forces researchers to communicate in laymen’s terms.
5. It is externally motivated by funders.
Bietrix warns it is important that patient engagement efforts should not just be window dressing, something she too often sees in how it is perceived or implemented by researchers.
Like with ELSI issues, availability of and access to useful tools and knowledge is a challenge. Therefore, EATRIS and partners developed the PERC as a central resource of available knowledge and expertise to help researchers to get started, understand the concept, and guide them to information. The PERC is a work in process: you can contribute by submitting a resource or story, Bietrix says. Her concluding remark:
"Patient engagement is by definition a co-production, a dialogue. And if you do it, do it right."
Florence on engaging with patients


Further details
This event, that connected researchers to research infrastructures, was the first of this format organised by Lygature. To request another, contact Jan-Willem Boiten.
It was made possible thanks to the EOSC-Life project that is funded from the European Union’s Horizon 2020 programme under grant agreement number 824087. You can read about events and follow the project's outcomes on the website.